Lithium-ion batteries have transformed the EV industry, but they don’t come without their disadvantages. You know, like the fact they sometimes cause EVs to burst into flames and explode.
LA startup Nanotech Energy claims to have found a way to eliminate this concern once and for all: a fireproof, graphene-based lithium-ion battery. And if this battery can do what it’s promised, we’re talking about a true game changer.

What is graphene?
Graphene is a single layer of carbon atoms, with a bunch of remarkable physical properties: it’s ultrathin, incredibly strong, superconductive, and cheap.
It was first discovered by in 2004 by two researchers at the University of Manchester, who managed to extract and isolate it from graphite. Although we reached out to the researchers for this article, they unfortunately declined to comment.
How has Nanotech used graphene to create batteries?
Nanotech has developed graphene electrodes, namely, the battery’s positive (cathode) and negative (anode) terminal.
According to the company, graphene’s flexibility means that it can withstand the volume changes of the battery electrodes during charge and discharge. This reduces the chances of an internal short circuit, which can potentially lead to fire.
What’s more, graphene is an excellent conductor of electricity, which helps the battery maintain a lower internal resistance — simply, the ability to maintain a lower internal temperature. As as result, it provides an efficient solution to overheating during charging, which is another potential cause of fire.
A novel non-flammable electrolyte
The electrolyte is a vital part of a lithium-ion battery, as it transfers lithium ions between the two electrodes during charge and discharge.
But… it’s the battery’s most flammable component.
Most electrolytes in use today involve dissolving a lithium salt in a liquid material composed primarily of linear and cyclic chain carbonates. Basically, molecules that involve a carbon atom attached to three oxygen atoms. These liquids are typically flammable, and can be volatile and unstable when exposed to high temperatures.
Nanotech has developed a proprietary electrolyte solution, called OrganoLyte, that is “stable, made from inexpensive materials, easy to manufacture and, of course, is non-flammable.”
While no specifics have been disclosed, the name suggests the material still centers around organic chemistry. We’ve reached out to the company to know more about it, and we’ll update the article when we have more information to share with you.
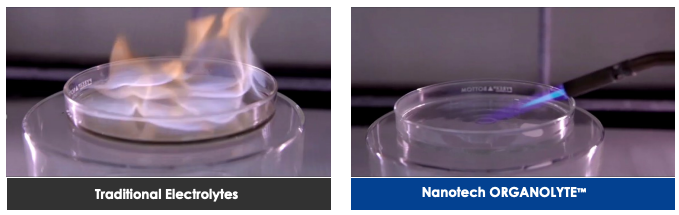
What have the tests shown?
Nanotech has run a series of tests to demonstrate that its graphene-OrganoLyte battery shows enhanced thermal stability and eliminates the risk of fire.
In the short video below, the company conducts a nail abuse test and a heating test at 180 degrees Celsius. In both, the standard lithium-ion battery used instantly catches fire, while the graphene battery remains unharmed.
A new super battery?
Testing has yielded some impressive results: the battery maintains performance at extreme temperatures (-20 to 60 degrees Celsius), it holds charge at temperatures as high as 176 degrees, and won’t catch fire when penetrated with a nail, or heated to more than 704 degrees.
But more than that, Nanotech also claims that its battery is extremely powerful. Namely, it retains more than 80% of its rated capacity through 1,400 cycles, and can charge “18 times faster than anything that is currently available on the market.”
Plus, the company’s new batteries can be “completely personalized to fit any form factor or container” — and can be used for consumer electronics, electric vehicles, and any electrified machines.
I guess, that’s why Nanotech’s batteries won the CES 2022 Innovation Award.
When will the tech be available?
Nanotech’s non-flammable lithium-ion batteries are set to be manufactured at a newly announced production plant in Nevada, scheduled to open in the fourth quarter of 2022.
I expect that initial production will focus on the consumer electronics market, as the batteries’ application in automotive use would require further durability, reliability, and safety testing. This might take some extra few years and I’m really looking forward to see the battery in action.
Get the TNW newsletter
Get the most important tech news in your inbox each week.